| |
|
 |
Pharmaceutical industry is a sunrise industry, especially the biopharmaceuticals, which just starts its large-scale industrialization process. Many large biotech and pharmaceutical companies are strengthening their presence worldwide. Compared with traditional pharmaceutical industry, biopharmaceutical industry sees a higher market concentration, and this is more helpful for strong enterprises to grow stronger and for building a number of leading enterprises in the segment areas. Currently, traditional chemical pharmaceutical makers also start turning their eyes towards biopharmaceutical field, and keep themselves busy in establishing much more strategic alliance.
China's pharmaceutical industry has been growing at 20% on the average in production value over the past decade, nearly doubled every four years. In Jan-Jul 2007, the accumulated production value of the entire industry reached RMB 342.449 billion, up 23.47% compared to the same period of last year, of which, production value of bio-pharmaceuticals was RMB 30.229 billion, up 24.37% from a year earlier.
Total Industrial Output Value and Growth, Jan-Jul 2007
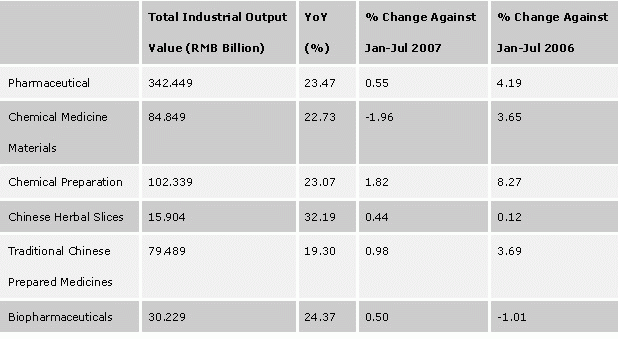
Source: State Information Center
China's biopharmaceutical industry could not only produce internationally important biologic drugs, but develop independently new biological drugs. Furthermore, China has achieved great breakthrough in gene engineering, biopharmaceuticals and clone technology. Despite of the rapid growth, there are still serious problems in China's biopharmaceutical industry:
China is weak in the investment, R&D capability and technology innovation;
There are serious repeated construction problems in drug development and production;
Overall, enterprises are small-sized, and with low modernization level and outmoded equipments;
Marketing strategies are unreasonable, mainly in brand development;
Management is outdated and talents capable of both technology and operation fall short;
There are no enough exchange and cooperation among enterprises.
Biopharmaceutical industry includes four segment markets: gene engineering, bacterin, blood products and diagnostic reagent. After more than a decade of development, these four segment markets find many aggressive private or state-owned biopharmaceutical enterprises through which China is to fulfill its supports to the whole industry. As far as capital market is concerned, as concept investment bubble fades away, Chinese pharmaceutical enterprises are expected to be gradually accepted by the market with its high growth potentials.
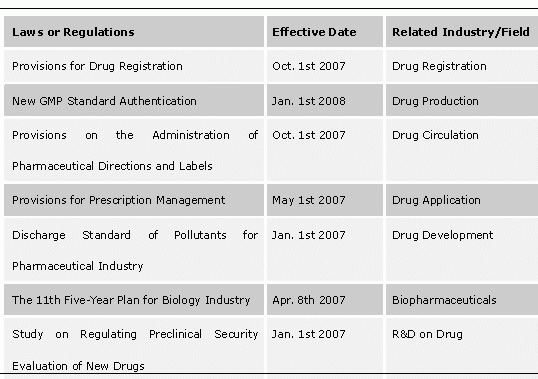
Polices have a great influence on China's biopharmaceutical industry, so how many efforts China will make to support the industry plays a significant role in its development. In fact, China always keeps a close eye on biopharmaceutical industry and has frequently issued related policies in recent two years, which is very positive for stimulating the development of biopharmaceutical industry and for regulating industry rules, and also brings energy and opportunities into the biopharmaceutical industry.
Related Laws and Regulations Issued in 2007
Based on the study on China pharmaceutical industry's status quo, competitive pattern, investment and developing trend, this report puts its emphasis on output and sales situation of main drugs, impact China's pharmaceutical industry will face as well as strategies enterprises should make.
|
|
|
|
|
If this report could not still meet your requirement, or
you have any comments or suggestions on it, please leave a
message to us.
|
2005-2007 www.researchinchina.com All Rights Reserved
| |
|
 |
| |
|
Pharmaceutical industry is a sunrise industry, especially the biopharmaceuticals, which just starts its large-scale industrialization process. Many large biotech and pharmaceutical companies are strengthening their presence worldwide. Compared with traditional pharmaceutical industry, biopharmaceutical industry sees a higher market concentration, and this is more helpful for strong enterprises to grow stronger and for building a number of leading enterprises in the segment areas. Currently, traditional chemical pharmaceutical makers also start turning their eyes towards biopharmaceutical field, and keep themselves busy in establishing much more strategic alliance.
China's pharmaceutical industry has been growing at 20% on the average in production value over the past decade, nearly doubled every four years. In Jan-Jul 2007, the accumulated production value of the entire industry reached RMB 342.449 billion, up 23.47% compared to the same period of last year, of which, production value of bio-pharmaceuticals was RMB 30.229 billion, up 24.37% from a year earlier.
Total Industrial Output Value and Growth, Jan-Jul 2007

Source: State Information Center
China's biopharmaceutical industry could not only produce internationally important biologic drugs, but develop independently new biological drugs. Furthermore, China has achieved great breakthrough in gene engineering, biopharmaceuticals and clone technology. Despite of the rapid growth, there are still serious problems in China's biopharmaceutical industry:
China is weak in the investment, R&D capability and technology innovation;
There are serious repeated construction problems in drug development and production;
Overall, enterprises are small-sized, and with low modernization level and outmoded equipments;
Marketing strategies are unreasonable, mainly in brand development;
Management is outdated and talents capable of both technology and operation fall short;
There are no enough exchange and cooperation among enterprises.
Biopharmaceutical industry includes four segment markets: gene engineering, bacterin, blood products and diagnostic reagent. After more than a decade of development, these four segment markets find many aggressive private or state-owned biopharmaceutical enterprises through which China is to fulfill its supports to the whole industry. As far as capital market is concerned, as concept investment bubble fades away, Chinese pharmaceutical enterprises are expected to be gradually accepted by the market with its high growth potentials.
Polices have a great influence on China's biopharmaceutical industry, so how many efforts China will make to support the industry plays a significant role in its development. In fact, China always keeps a close eye on biopharmaceutical industry and has frequently issued related policies in recent two years, which is very positive for stimulating the development of biopharmaceutical industry and for regulating industry rules, and also brings energy and opportunities into the biopharmaceutical industry.
Related Laws and Regulations Issued in 2007

Based on the study on China pharmaceutical industry's status quo, competitive pattern, investment and developing trend, this report puts its emphasis on output and sales situation of main drugs, impact China's pharmaceutical industry will face as well as strategies enterprises should make. |
|
|
|
|
2005-2006 www.researchinchina.com All Rights Reserved |
|
| |
|
 |
| |
|
1 Operation Status of China Pharmaceutical Industry in 2007
1.1 Overview
1.2 Problems in China pharmaceutical market
1.2.1 Competition in production is rampant
1.2.2 Capability in innovation is poor
1.2.3 Drug quality appears thread-and-thrum
1.2.4 Financing channel is undiversified and investment is in short
1.2.5 Drug circulation market is unorderly
1.3 Policies adjustment in China's pharmaceutical industry in 20072 Overview of Biopharmaceutical Industry
2.1 Brief introduction to biopharmaceutical industry
2.1.1 Definition
2.1.2 Development history
2.2 Main products and characteristics of biopharmaceutical industry
2.2.1 Main products
2.2.2 Characteristics
2.3 Development status of global pharmaceutical industry
2.3.1 USA
2.3.2 Europe
2.3.3 Japan
2.3.4 India 3 Environments in China Biopharmaceutical Industry
3.1 Macro environments
3.1.1 General situation of macroeconomic in China, 2007
3.1.2 Economic operations of China pharmaceutical industry
3.1.3 Influences caused by variational macro environments in China medical treatment field
3.2 Policy environments
3.2.1 Drug registration
3.2.2 Drug production
3.3.3 Drug circulation
3.3.4 Drug application
3.2.5 Supervision on sub-industries 4 Status Quo of China Biopharmaceutical Industry
4.1 Operations
4.2 Production technology
4.3 Major problems
4.3.1 Enterprises operate mostly at a small size
4.3.2 Repeated construction problems are serious
4.3.3 R&D capability is weak
4.3.4 Industry concentration is at a low level
4.3.5 Basic R&D results are unable to be converted efficiently 5 Industrial Systems in China Biopharmaceutical Industry
5.1 Biopharmaceutical industry systems
5.2 Problems exist in China biopharmaceutical industry systems
5.3 Value chain analysis in biopharmaceutical industry 6 Main biopharmaceutical products
6.1 Blood products
6.1.1 Operations status
6.1.2 Main blood products
6.1.3 Competitiveness
6.2 Bacterin
6.2.1 Overview of Bacterin market
6.2.2 Polices on immunity plan promote bacterin market to grow fast
6.2.3 Market competitiveness
6.2.4 Segment markets 7 Listed Companies
7.1 Hualan Biological Engineering Inc.
7.1.1 Company Profile
7.1.2 Operations in 2007
7.1.3 Opportunities and risks
7.2 Shanghai Kehua Bio-engineering Co., Ltd
7.2.1 Company profile
7.2.2 Operations in 2007
7.2.3 Risks
7.3 Beijing Tiantan Biological Products Co., Ltd
7.3.1 Company profile
7.3.2 Operations in 2007
7.3.3 Opportunities and risks
7.4 Da An Gene Co., Ltd of Sun Yat-Sen University
7.4.1 Company profile
7.4.2 Operations in 2007
7.4.3 Opportunities and risks 8 Foreign Companies in China
8.1 Bayer
8.1.1 Operations in China
8.1.2 Investments in China
8.2 AstraZeneca
8.3 Pfizer
8.3.1 Operations in China
8.3.2 Investments in China
8.4 Sanofipasteur
8.5 Novartis
8.6 GlaxoSmithKline (GSK)
8.7 Lilly
8.8 Co-operative Group
|
|
|
|
|
2005-2006 www.researchinchina.com All Rights Reserved |
|
| |
|
 |
| |
|
Revenue and Profit Growth of Pharmaceutical Industry by Segment, Jan-Aug 2007
Growth of Sales Revenue and Gross Profit of Pharmaceutical Industry
Per Capita GDP and Medical Care Depending/GDP Worldwide
Forecast on Trend of Proportion of Total Health Expenditure to GDP in China
Total Sum Spent by Sample Hospitals on Albumin Market
Ranking of Drugs Used by Hospitals by Purchase Sum, 2006
Price Trend of Albumin
Product Structure of Blood Products Market in China, 2006
Product Structure of Blood Products Market in the World, 2006
PCC Market Share by Manufacturer, 2006
IVIG Market Share by Manufacturer, 2006
HRIG Market Share by Manufacturer, 2006
List of Blood Products Manufacturers in China
Total Amount of Plasma in China
Product Structure of Key Blood Products Manufacturers
Share of Sample Hospitals in Albumin Market, 2006
Main Brands of Imported Human Serum Albumin Injections in China
Newly-increased Bacterins after Starting the Expanded Program on Immunization
Number of Manufacturers by Main Bacterin
Key Projects in Process
Main Business Revenue and Gross Profit Margin by Company
Profitability Indices by Company
Indices of Operating Ability and Debt-Paying Ability by Company
Product Structure Change by Company
Shareholding Structure of Da An Gene Co., Ltd of Sun Yat-Sen University
Financial Indices of Listed Diagnostic Reagents Companies, 2007H1
Forecast on Da An Gene's Diagnostic Reagents Development
Key PCR Reagents Manufacturers in China
Comparison of In-Vitro Diagnostic Methods
Catalog of PCR Reagents Reported by Da An Gene to State Food and Drug Administration, 2006-2007
Penetration Status of Medical Insurance System
Population and Structure in China
Engel's Coefficient Change in China
Per Capita Health Expenditure in China, 2000-2004
Major Laws and Regulations Issued in 2007
Market Capacity of Segmented Blood Products
Supervision Policies on Blood Products
|
2005-2008 www.researchinchina.com All Rights Reserved
|



